Abstract
Purpose Axicabtagene ciloleucel (axi-cel, Yescarta® (Axi)) and tisagenlecleucel (tisa-cel, Kymriah® (Tisa)) have been approved and commercialized in Europe for Relapsed/Refractory Diffuse Large B-Cell Lymphoma (DLBCL) after 2 or more previous lines of therapy. A recent Lysa Study from a matched analysis of the Descar-T registry has reported that Axi may yield a higher disease control rate compared to Tisa but at the expense of a higher toxicity[Bachy et al. EHA 2022]. Biological reports have shed light on the importance of both expansion and persistence of CAR T-cells to enhance disease control. In addition, in a mouse model, viability levels of CAR T cells both immediately after thawing and after 1 day of in-vitro culture were reported to be important indicator of the CAR-T cells antitumor activity [Zah E et al. Can Im Res. 2016]. In this single-center prospective study, we analyzed biological characteristics of both Axi and Tisa anti-CD19 CAR T-cells infused in 48 patients and looked at their correlation with outcomes.
Experimental design From March 2019 to June 2022, residual cells were obtained after washing 48 infusion product bags, 27 Axi and 21 Tisa products. Cells' viability was analyzed immediately, and percentage of CAR positive T-cells was determined by flow cytometry with CD19 CAR detection reagent (Miltenyi Biotec). Cells were put in complete medium (RPMI, glutamax, 8% of human serum and 300 UI of IL-2/ml) at 37°C, viability was analyzed after 1 days of in-vitro culture and doubling population number was followed for 1 to 4 weeks.
Results Among the 48 patients (median age: 62 yo, range: 37-78), 56% had a DLBCL, and 29% had a transformed Follicular Lymphoma. Median number of previous lines was 3 (range, 2-10), which included an ASCT in 37.5% of patients. No differences in terms of characteristics were observed between Tisa and Axi cases.
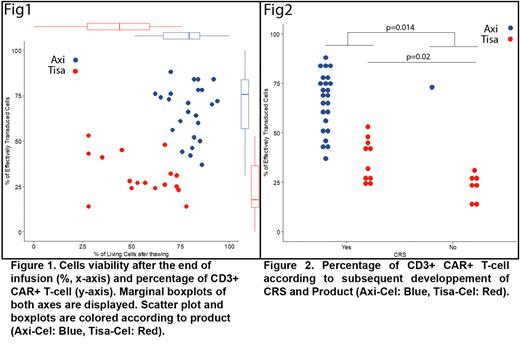
Tisa displayed a lesser cell viability (median 57%, range: 28-78) as compared to Axi (81%; range 62-94, p<0.001, (Figure 1). After 24H, the median percentage (%) of live cell recovery were similar (Tisa 89%, vs Axi 84%; p=0.23). However, 43% of Tisa and 20 % of Axi preparations displayed a viable cell yield ≤ 50% the day after thawing. The recommended doses of viable CAR+ T cells to be injected in patients is 0.6 to 6.0 x 108 cells for Tisa and 2 × 106/kg, with a maximum of 2 × 108 cells, for Axi. All patients received the recommended dose even if Tisa products were characterized by a significantly lower fraction of CD3+ CAR+ T-cells as compared to Axi (median 27% , range: 14-53 vs. 70%; range 37-88 P<0.001) (Figure 1). Median CD4/CD8 ratii among CAR+ T cells were similar between both products (Tisa: 3.5 vs Axi 2.3, p=0.35). In vitro and in the absence of antigen stimulation, total T-cells were able to proliferate, Tisa product had a higher amplification capacity (median nbr of division = 8.8 vs 3.1, p=. 6e-6). Finally, the proportion of CAR negative T cells (mostly activated T-cells) injected was highly variable between patients and ranged from 1.3.108 to 61.4.108 for Tisa while it was only from 0.2.108 to 3.4.108 for Axi.
All but one patient infused with Axi presented with cytokine release syndrome (CRS) compared to 62% of patients infused with Tisa (p=0.006); the same significant difference was observed for immune effector cell-associated neurotoxicity syndrome (ICANs) (67% vs 14%, p<0.001). Conversely, survivals were comparable in this small cohort. Only the percentage of CD3+ CAR+ T-cells had a clinical impact. Indeed, a significant higher median % of these cells was found in patients developing all grade CRS (58% [range, 24-88%] vs 25% [range, 14-73%], p=0.014). (Figure 2) This was also true when considering only patients receiving Tisa (CRS+ 36.5 % [range, 24-88%] vs CRS- 24% [range, 14-73%], p=0.02. No biological association with ICANs nor hematological toxicity could be reliably reported in this cohort.
Discussion In this study, four major biological differences were observed between Tisa and Axi products: the cell viability, the proliferative activity in vitro, the % of CAR positive T-cells and the total amount of CAR negative T-cells injected into the patient. However, impact on outcomes was modest as only CRS were found to be correlated to the percentage of CD3+ CAR+ T-cells injected, of note Axi-cell which was reported to be associated with a better disease control rate developed more CRS in our cohort, which is line with a high and consistent percentage of CD3+ CAR+ T-cells.
Disclosures
Gastinne:Gilead/Kite, Takeda: Honoraria, Membership on an entity's Board of Directors or advisory committees, Other: Support for attending meetings/travel, participation in a data safety monitoring board or advisory board. Touzeau:BMS: Honoraria, Membership on an entity's Board of Directors or advisory committees. Moreau:AbbVie, Janssen, Celgene, Amgen, and Sanofi: Honoraria. Le Gouill:Novartis, Kite/Gilead, Janssen: Honoraria, Membership on an entity's Board of Directors or advisory committees, Other: Travel support. Chevallier:Incyte: Research Funding; Jazz Pharmaceuticals: Honoraria; Pfizer: Research Funding; Abbvie: Honoraria; Takeda: Honoraria.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal